Indicating Electrolysis
Breaking up (water) isn't hard to do.
Break up water into hydrogen and oxygen gas with a simple electrolysis device, and use an acid-base indicator and a magnet to create groovy swirls of color.


- Two stainless steel screws, each at least 1.5 inches (40 millimeters) long (available at your local hardware store)
- Shallow clear glass or plastic container such as a Petri dish
- Epsom salts (available at your local drugstore) CAUTION: don't substitute table salt for this activity because it can produce chlorine gas
- 9-volt battery
- Rubber bands
- Acid-base indicator such as phenol red, phenolphthalein, bromothymol blue, or cabbage juice indicator (see the Going Further section below for instructions on how to make your own)—make sure you’re aware of any safety issues and that you know which colors indicate acid and/or base when using your chosen indicator
- Safety goggles
- Spoon
- Optional: magnets

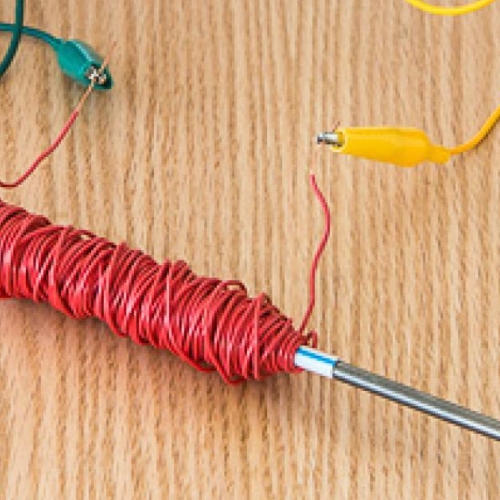
- Wrap a rubber band lengthwise around the 9-volt battery so that the rubber band lays across both of the battery terminals. Wrap another rubber band lengthwise around the battery so it crosses the first rubber band in a perpendicular fashion.
- Slide a stainless steel screw beneath the rubber band and on top of each battery terminal. Each screw should be positioned so that its tread is in contact with the terminal. The heads of the screws should point in the same direction and the screws should not touch each other (see photo). This is your electrolysis device, and the screws are your electrodes—they’ll be conducting electricity during the experiment.
- Put on your safety goggles.
- Fill your Petri dish or shallow container with lukewarm water.
- Pour Epsom salt crystals into the water (about a 1/3 teaspoon if you’re using a Petri dish). Stir your solution with a spoon or your finger to help dissolve the salt.
- Add some of your chosen acid-base indicator to the salt solution until the solution is colored—you’ll need from a few drops to a few milliliters depending on the type and the concentration of your indicator.
Submerge the exposed ends of the screws in the solution. Make sure the battery itself doesn’t get wet! You should be able to rest your electrolysis device on the edge of the container.
What do you notice happening at the submerged ends of each screw? Your screws should begin emitting gas bubbles, and different colors should develop at each electrode.
Did you see bubbles forming at the screw tips? Did one screw emit more bubbles than the other? Look and see which battery terminal is associated with which volume of bubbles, and which terminal is associated with which indicator color.
The molecular formula for water is H2O, where H stands for the element hydrogen and O stands for the element oxygen. In a glass of water, many of the molecules naturally separate out into hydrogen ions (H+) that are positively charged and hydroxide ions (OH-) that are negatively charged. Your electrolysis device causes reactions that pull apart the water even more.
Since opposite charges attract, the hydrogen ion migrates towards and bubbles from the negative electrode and the oxygen-containing hydroxide ion migrates towards and bubbles from the positive electrode. At the same time, you also create a change in pH, or the amount of acid and base, in local areas around the electrodes.
At the positive electrode, two oxygen atoms get pulled from two hydroxide ions and combine to make oxygen gas (O2). This leaves an abundance of free hydrogen ions, which makes it more acidic at the positive terminal—so your indicator should show an increase of acid at the positive terminal. Likewise, when hydrogen ions combine to make hydrogen gas (H2) at the negative terminal, an abundance of freed hydroxide ions are produced near the negative terminal. Therefore, your indicator should show the production of a base at the negative terminal.
The Epsom salt, also know as magnesium sulfate (the chemical formula for magnesium sulfate is MgSO4), is dissolved in the water to help your battery break up the water more efficiently. Epsom salt breaks up into ions, and these charged particles help carry the electric current around the solution.